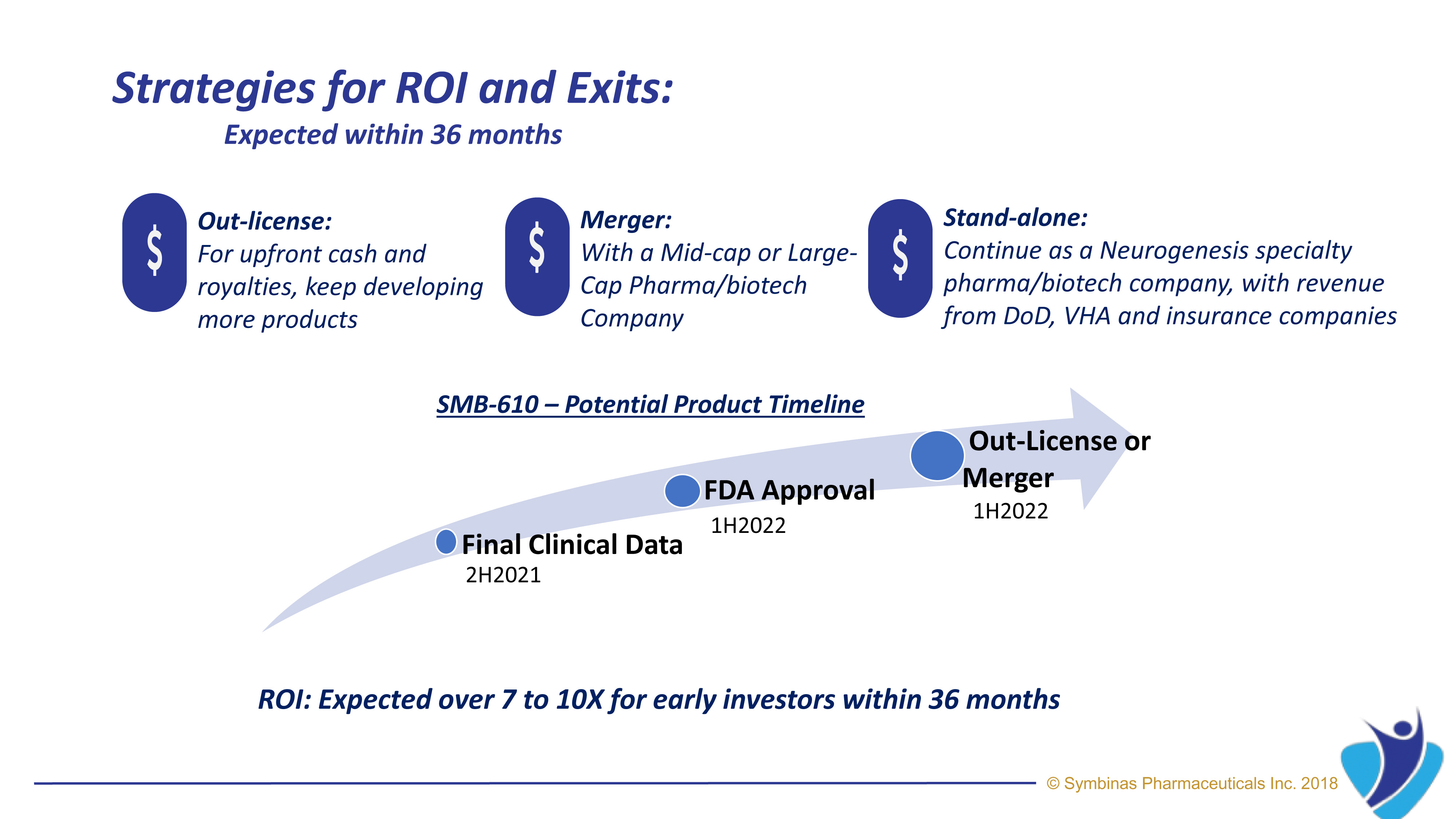
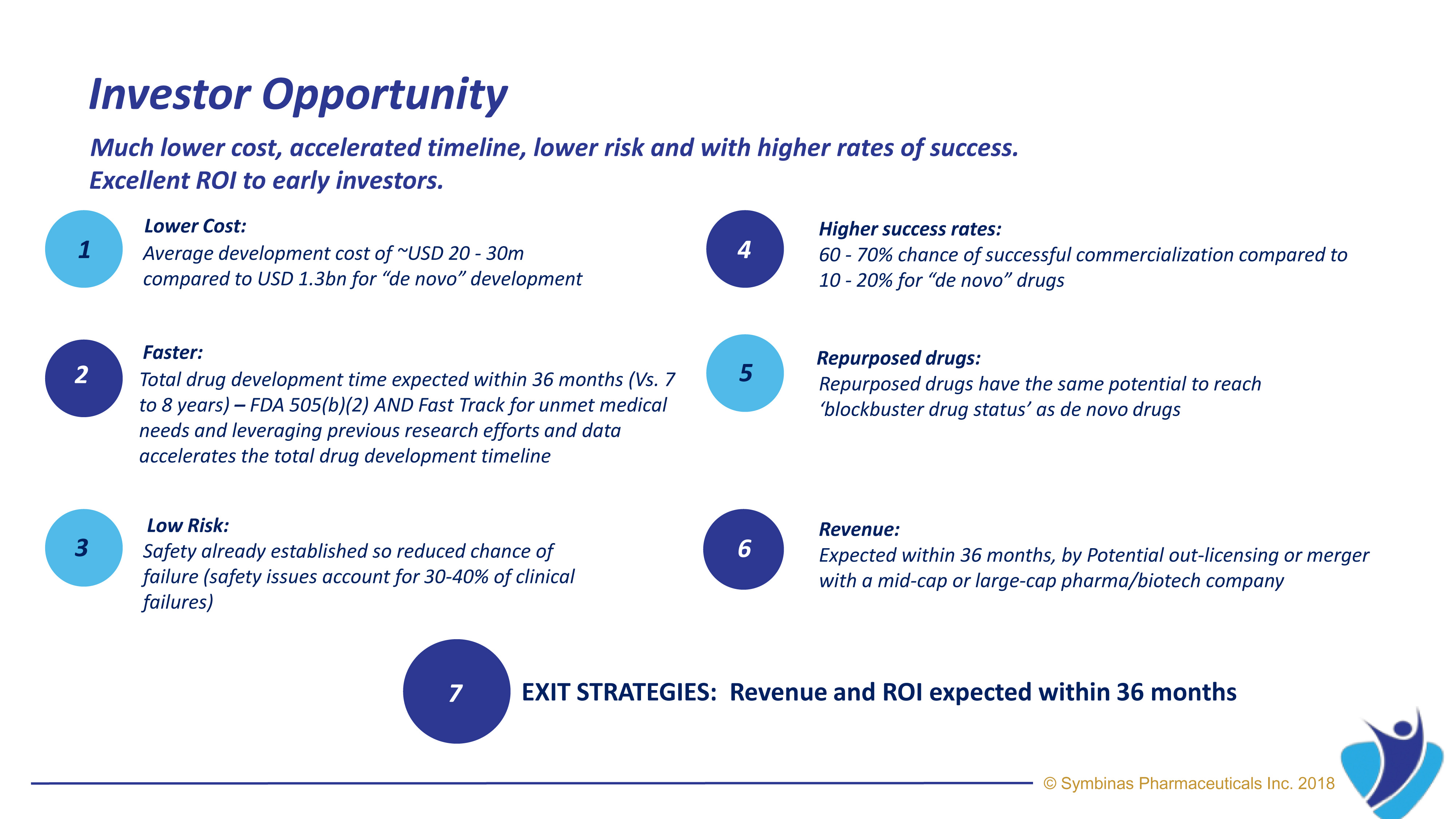
The role of mitochondria in cellular energy metabolism, cell signaling, and aging is well established. Mitochondrial dysfunction in cerebral tissue is a center phenomenon during post-traumatic neurometabolic cascade occurring after primary damage from concussion and TBI.
The neurological consequences of mitochondrial impairment including neuronal apoptosis, increased fission, axonal injury, production of oxygen free radicals and Blood-brain-barrier disruption are well documented. There is also evidence for potentially worse mitochondrial dysfunction associated with repetitive TBI compared with single mild TBI.
Mitochondrial function is intimately linked to cellular survival, growth, and death. The role of mitochondria in cellular energy metabolism, cell signaling, and aging is well established. Mitochondria are the powerhouses and the major source of free radicals in most cells including neurons and glial cells in brain.
Mitochondrial dysfunction has been implicated in many neurological diseases, including Parkinson’s disease, Alzheimer’s disease, stroke and TBI. Other than ATP production, mitochondria participate in numerous other cellular events including intracellular calcium buffering, generation of reactive oxygen species (ROS), and apoptosis.
It is well established that adult neurogenesis is critical for recovery from brain injury. And that cellular mitochondria are essential for the differentiation of neural stem cells into mature neurons. Inflammatory responses from activated glial cells after brain injury cause mitochondrial damage in newly generated neurons, leading to significant loss in neurogenesis at the injury site. Improving cellular mitochondrial function and decreasing mitochondrial damage from inflammatory response leads to improved neurogenesis.