Home / PRODUCTS
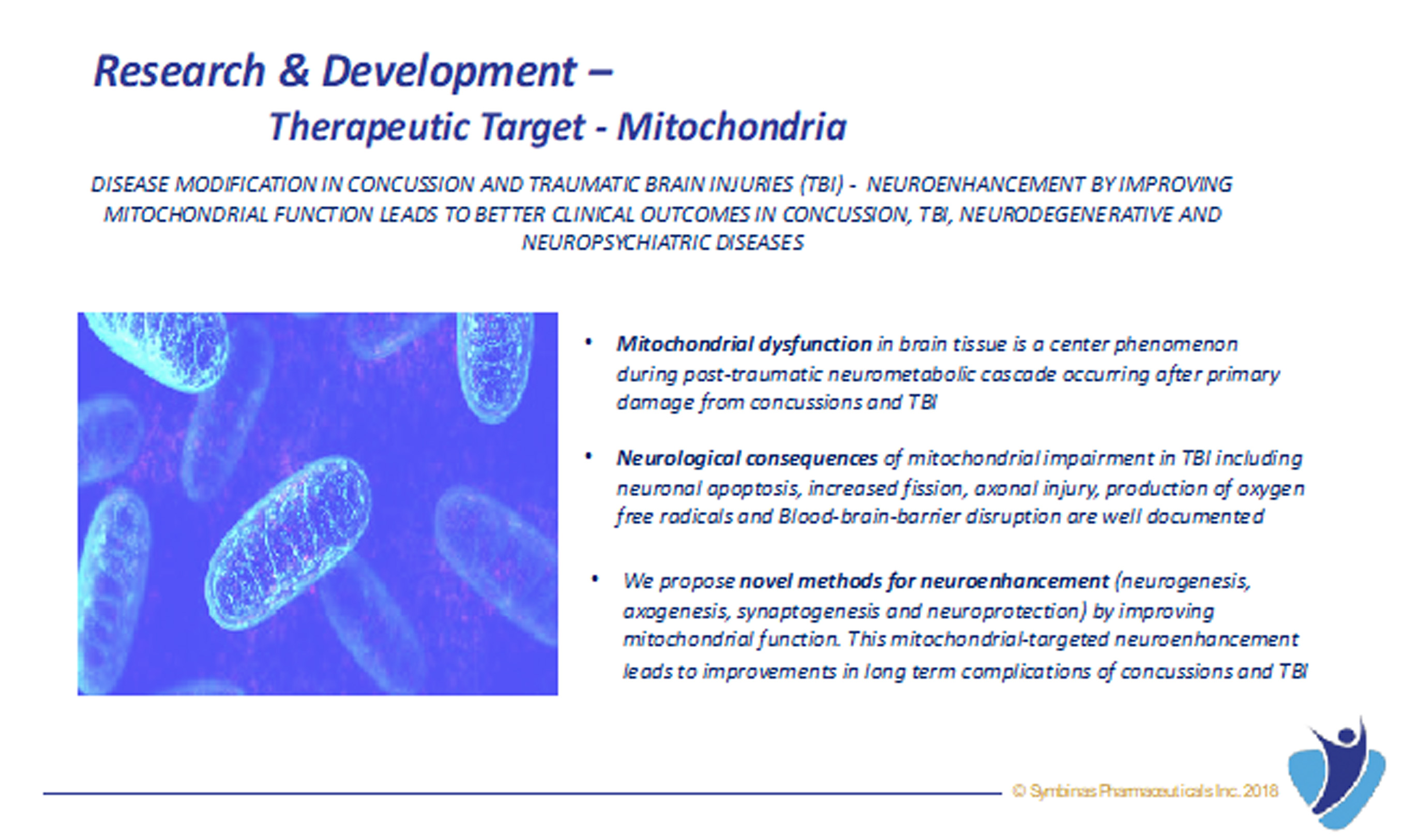
By improving mitochondrial function and inhibiting neuro-inflammation, our drug candidates treat the real cellular causes behind concussions and TBI (and NOT simply controlling symptoms) and thus provide treatment for long-term complications of concussions and TBI, such as anxiety, PTSD, insomnia, dementia, agitation, and possibly even Alzheimer’s and Parkinson’s disease.
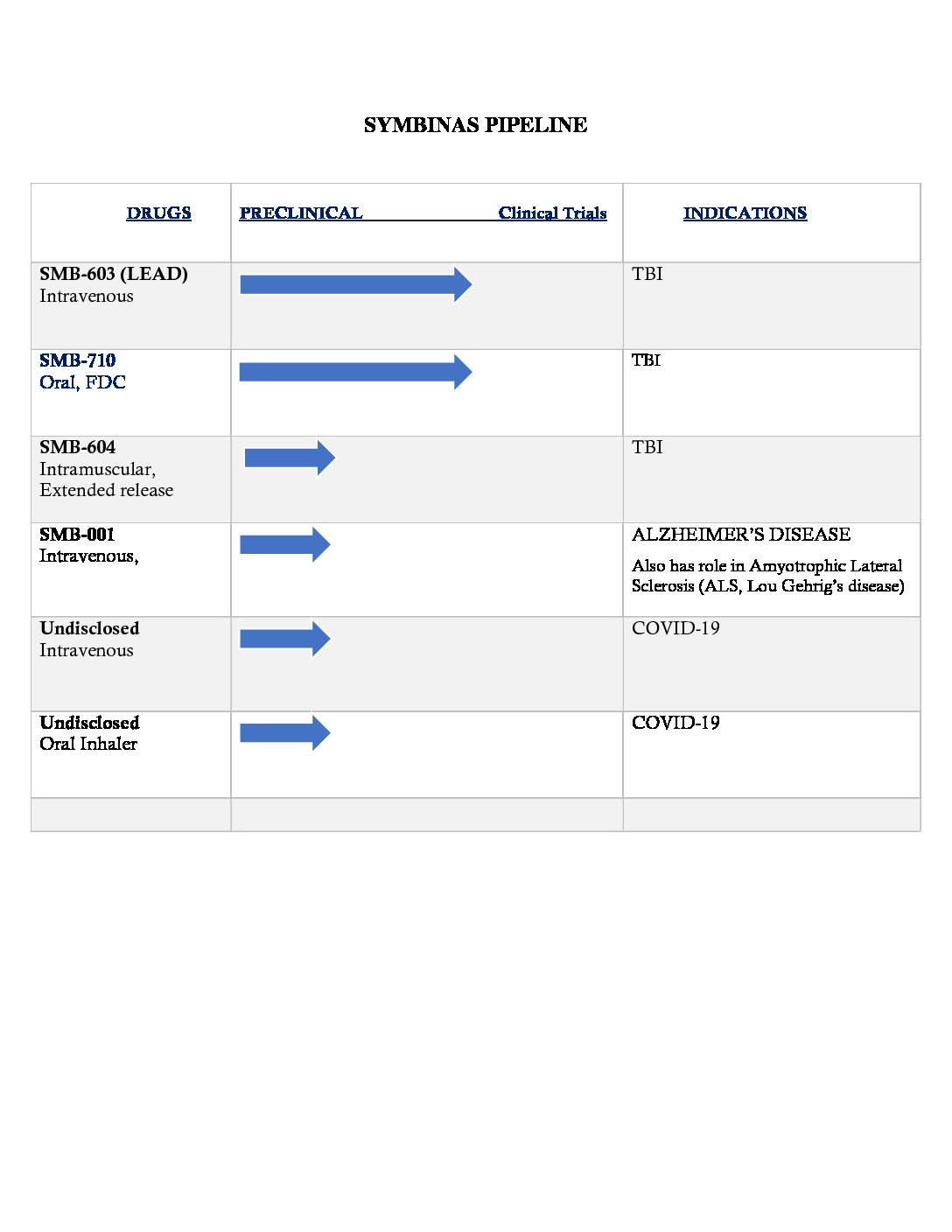
Symbinas has expansive pipeline of dug development candidates including Two Drug Candidates To Treat Concussion And TBI. Both of these drug candidates are being developed for FDA’s unique pathways including Fast-track submissions.
Symbinas has TWO Fast-Track drug product candidates ready for clinical development for TBI:
- LEAD Candidate: SMB-603 is Symbinas lead drug candidate for TBI. This is being developed with approval target date of 24 – 36 months. Also has possible role in Alzheimer’s disease and other dementia.
- Symbinas is also developing SMB-710 for TBI.
- SMB-710 has use in Alzheimer’s and Parkinson’s disease.
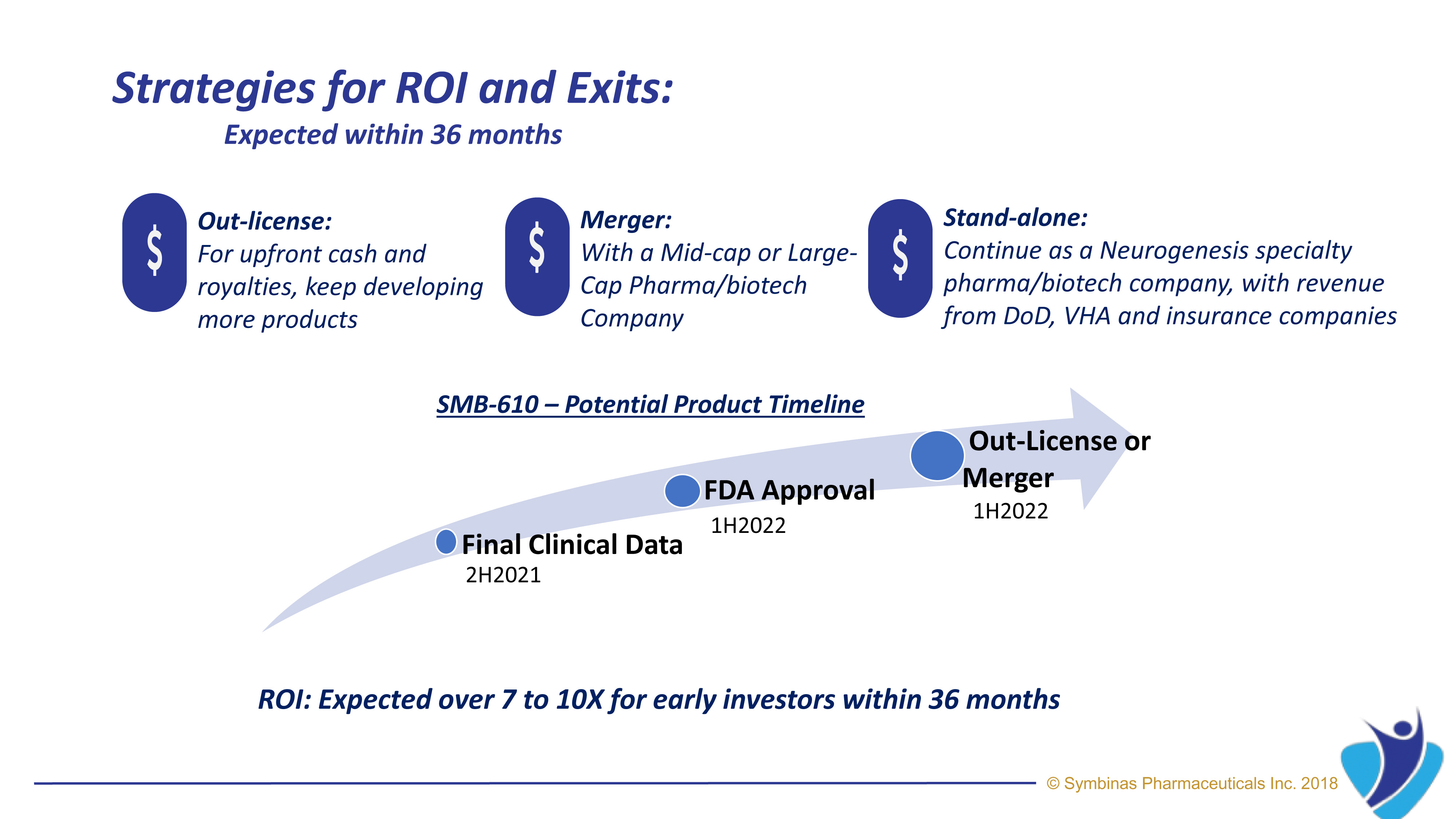
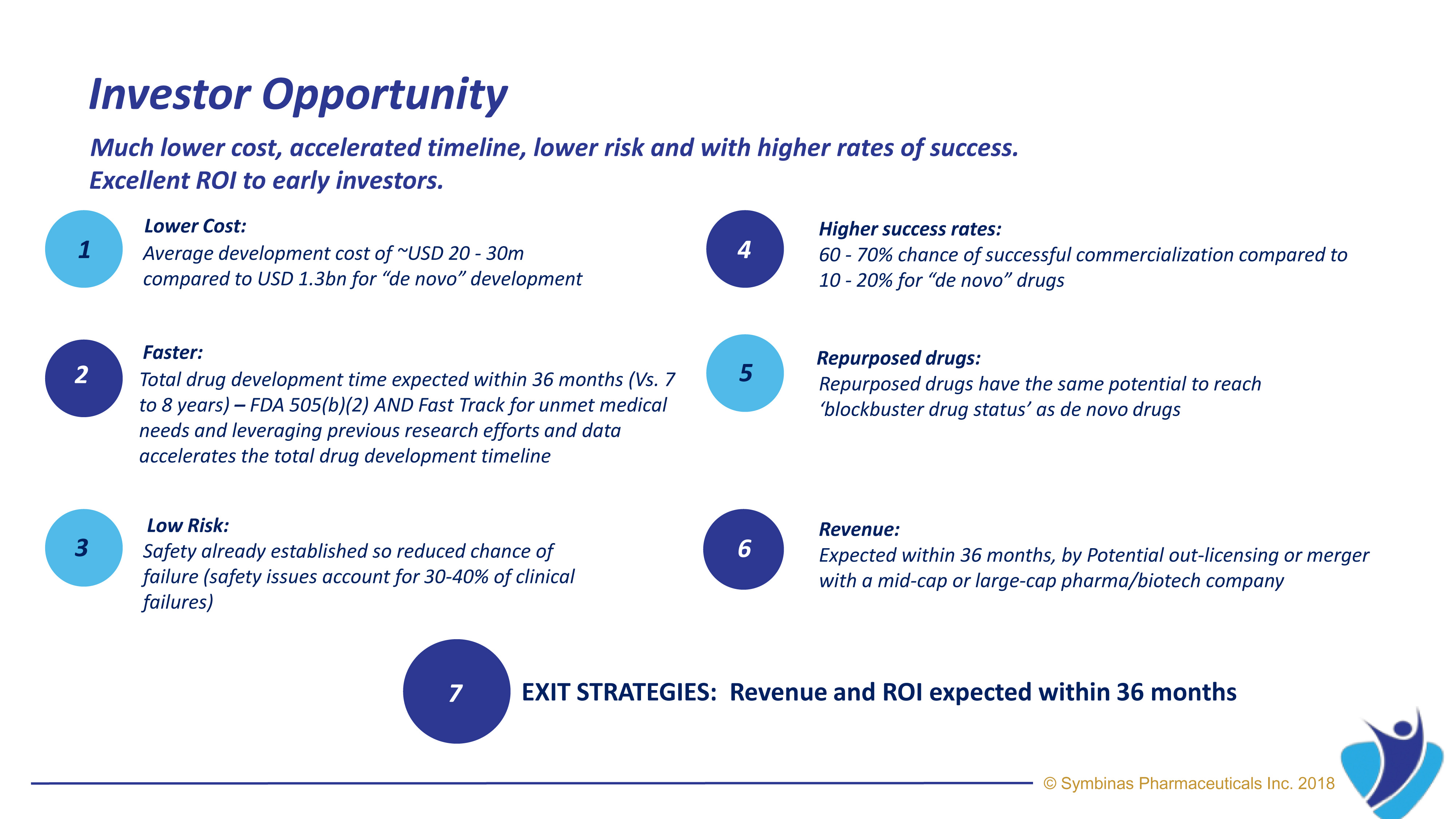
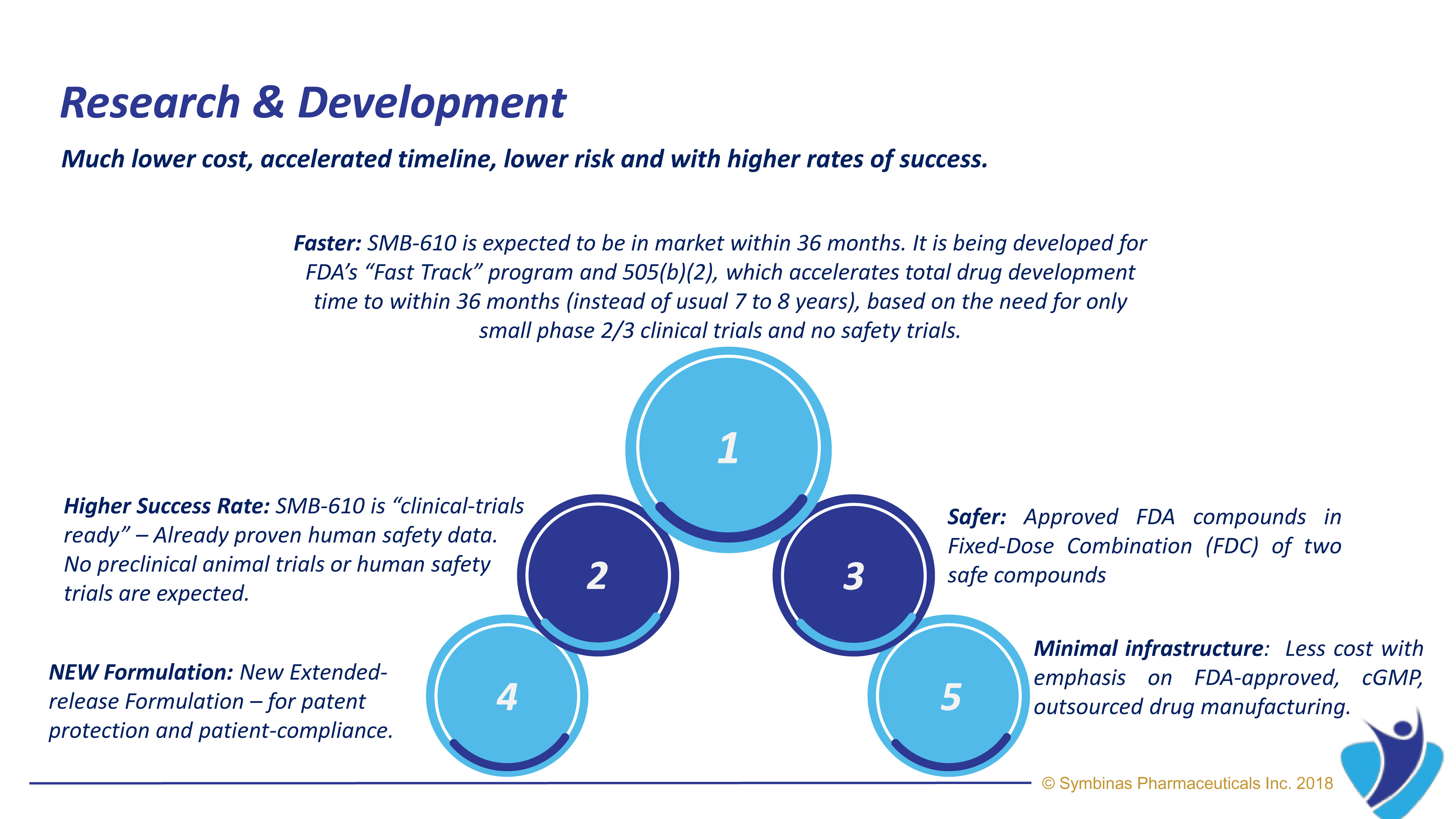
Strategic Regulatory Pathways provide much lower cost, accelerated timeline, lower risk and with higher rates of success. Using FDA’s special Fast-Track Pathway and Emergency Use Authorization (EUA)
SMB-603, LEAD PRODUCT
- Mode of Action – Mitochondrial enhancement and stem cell stimulation by SMB-603 repairs the cellular damage seen in concussion and TBI.
- Expected to treat the brain damage, and not just symptoms, caused by concussions and TBI, and therefore has the potential to transform the lives of millions of patients WORLDWIDE.
- Data – Existing Data – It already has proven human safety data and has been found to be effective in human case treatments as well as in vivo models for receptor targets.
- It is expected to qualify for FDA’s special Fast-track submissions for Concussion and TBI
SMB-710
- Mode of Action – Inhibiting Neuroinflammation
- Potential uses include Alzheimer’s disease and Parkinson’s disease
- It is Fixed-dose combination of two FDA-approved ingredients
- Existing Data – It already has proven human safety data and has been found to be effective in human case treatments as well as in vivo models for receptor targets.
- It is expected to qualify for FDA’s special Fast-track submissions
EXPANSIVE PIPELINE OF DRUG CANDIDATES
BASED ON SCIENTIFIC DATA