Home / About Us

OUR STORY
“Why there is still no treatment for concussion?” asked Dr. Hardesh Garg after one of his own family members had concussion and the only treatments given for this serious illness were supportive such as bed rest and pain medicines. Finding that in past decades no real advancement has been made in treating this common and serious disease, he launched Symbinas in 2018 with the purpose of finding treatment for Traumatic Brain Injury. So began our journey to develop novel therapeutics for this global crisis.
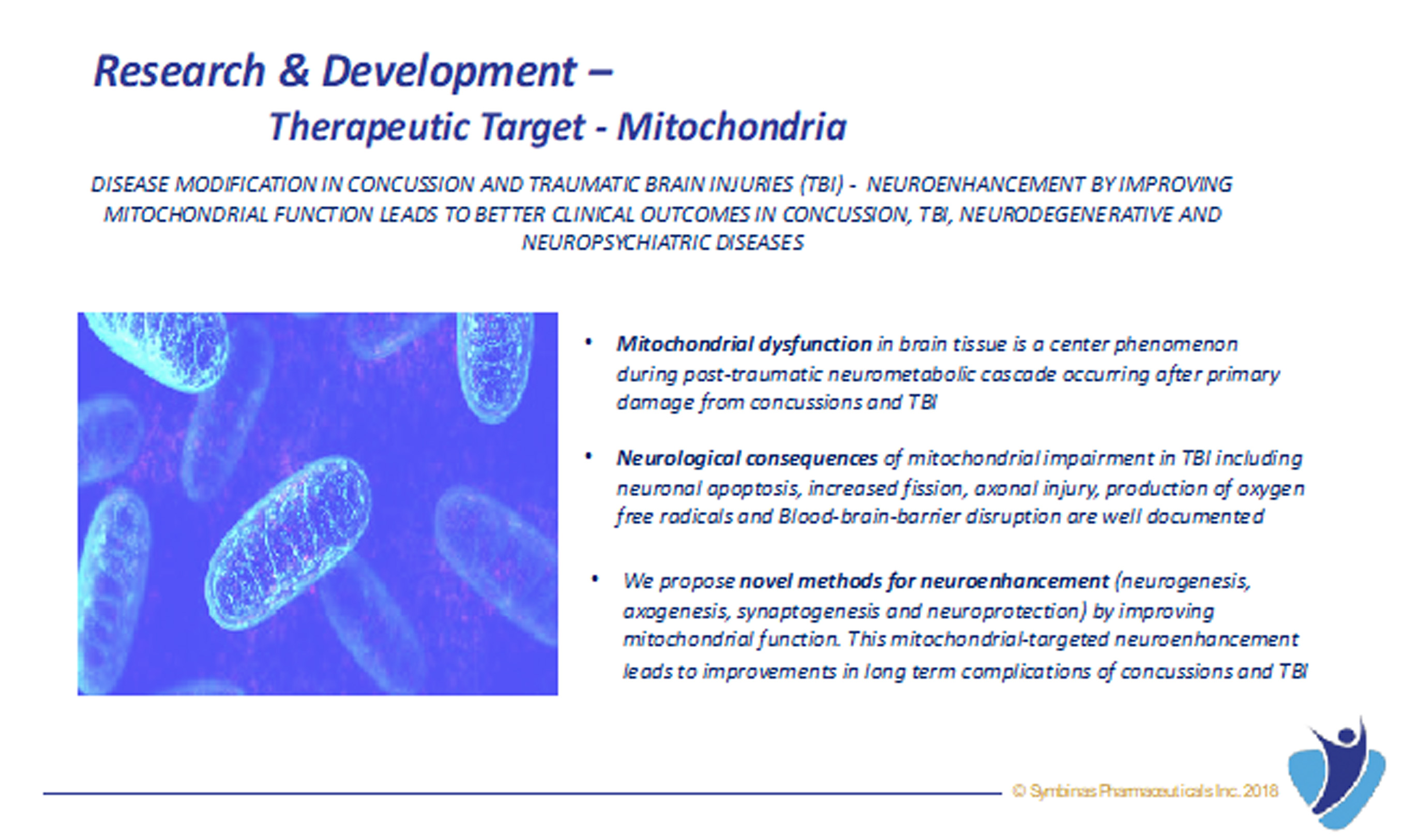
Relying on his decades of experience as physician and researcher, he began to methodically review an endless supply of scientific literature and understand molecular advances in TBI from research scientists at top academic institutions around the world. He also gained tremendous clinical insight from his own patients, many were retired NFL athletes and war veterans, and from other healthcare providers engaged in treating TBI patients. Dr. Garg then decided the best way to prevent worsening in this disease was to focus on improving mitochondrial function and to inhibit neuro-inflammation that will improve and preserve brain function.
What started as a simple question out of concern for his own family member is now becoming a reality. Symbinas is advancing its lead drug candidate, SMB-603, into intravenous formulation development and plans to start clinical trials in patients with TBI next year.
Every day at Symbinas we’re working to turn TBI from rapidly worsening condition for millions of people globally into one that can be managed, and even cured, medically. Together we will rewrite what’s possible.
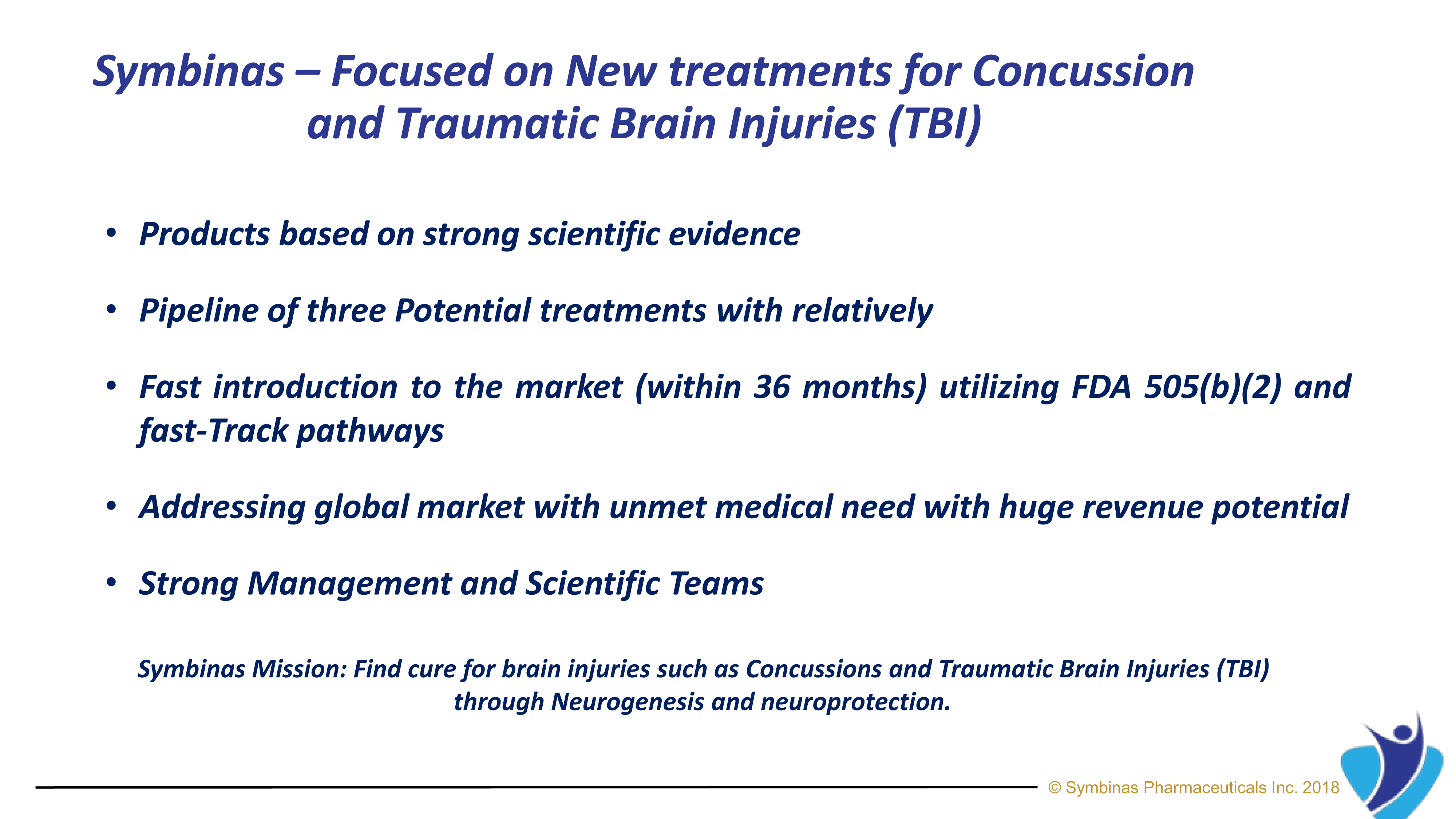
Our Mission Is To Find Cure For Concussions And Traumatic Brain Injuries Through Neurogenesis And Neuroprotection.
ABOUT US
Symbinas Pharmaceuticals Inc. (“Symbinas”) is a privately held, U.S., early-stage biopharmaceutical company focused on developing therapeutics to make significant differences in the lives of patients with unmet medical needs worldwide. It is now developing SMB-603 and SMB-710 to treat TBI.
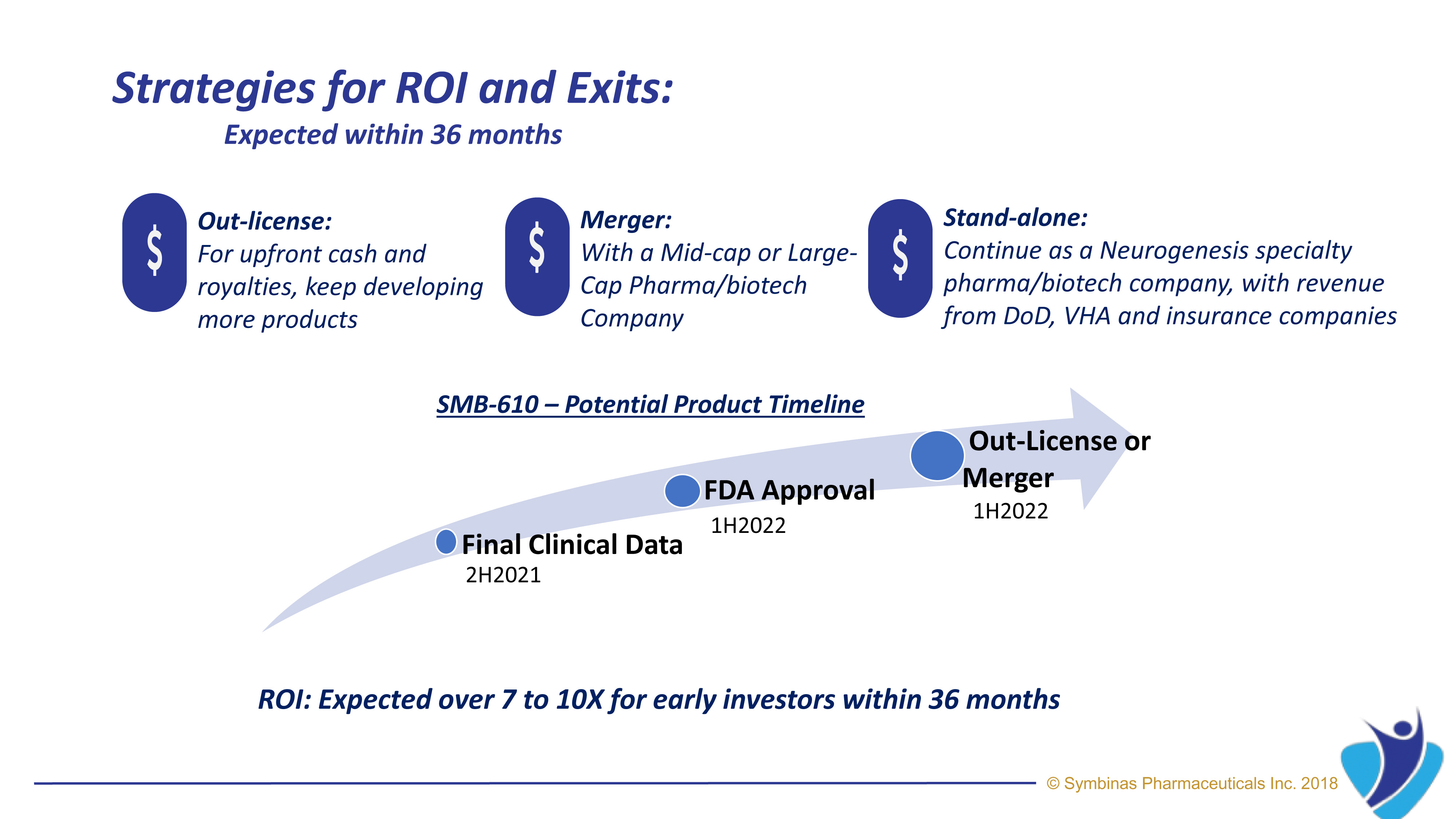
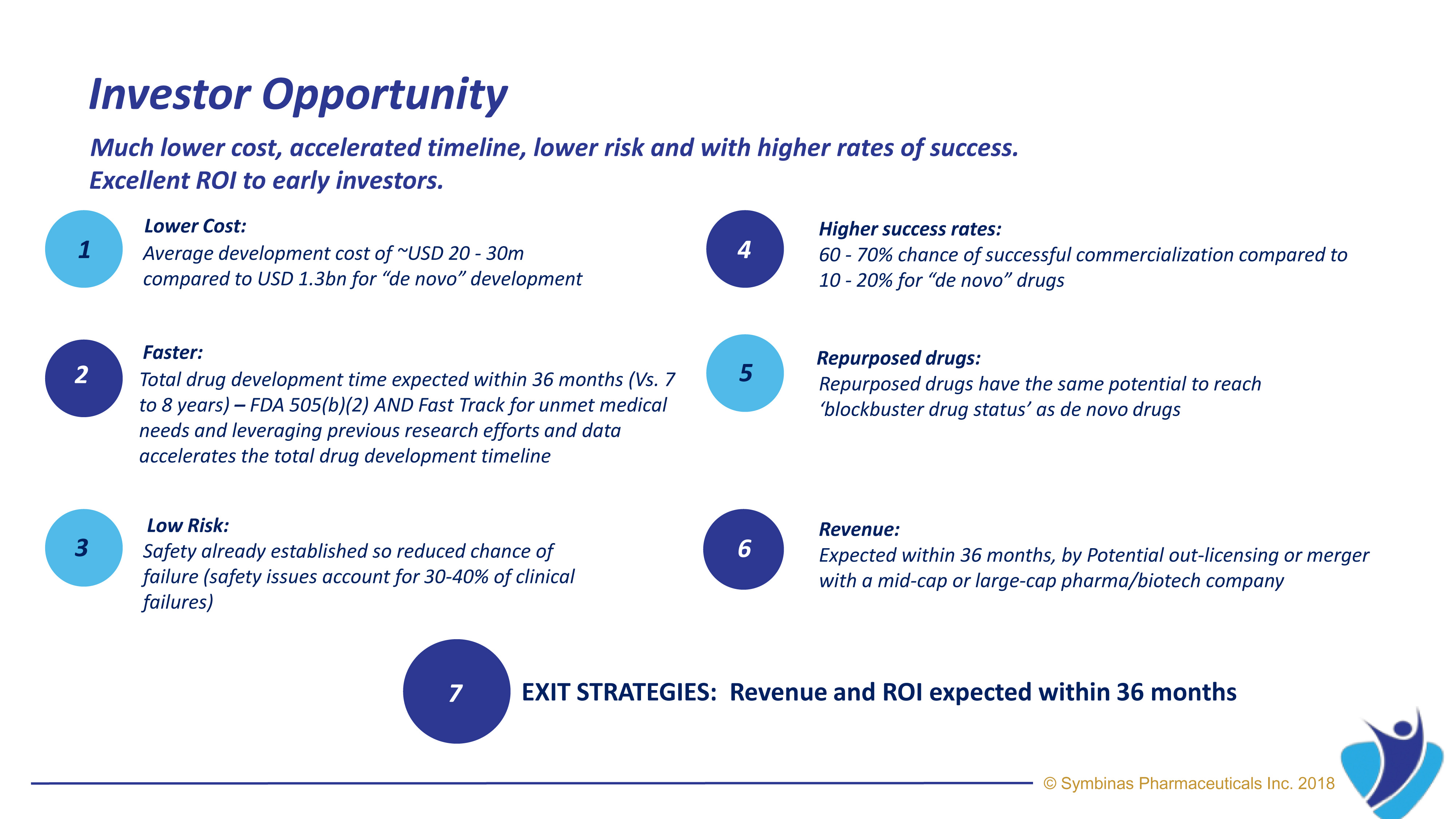
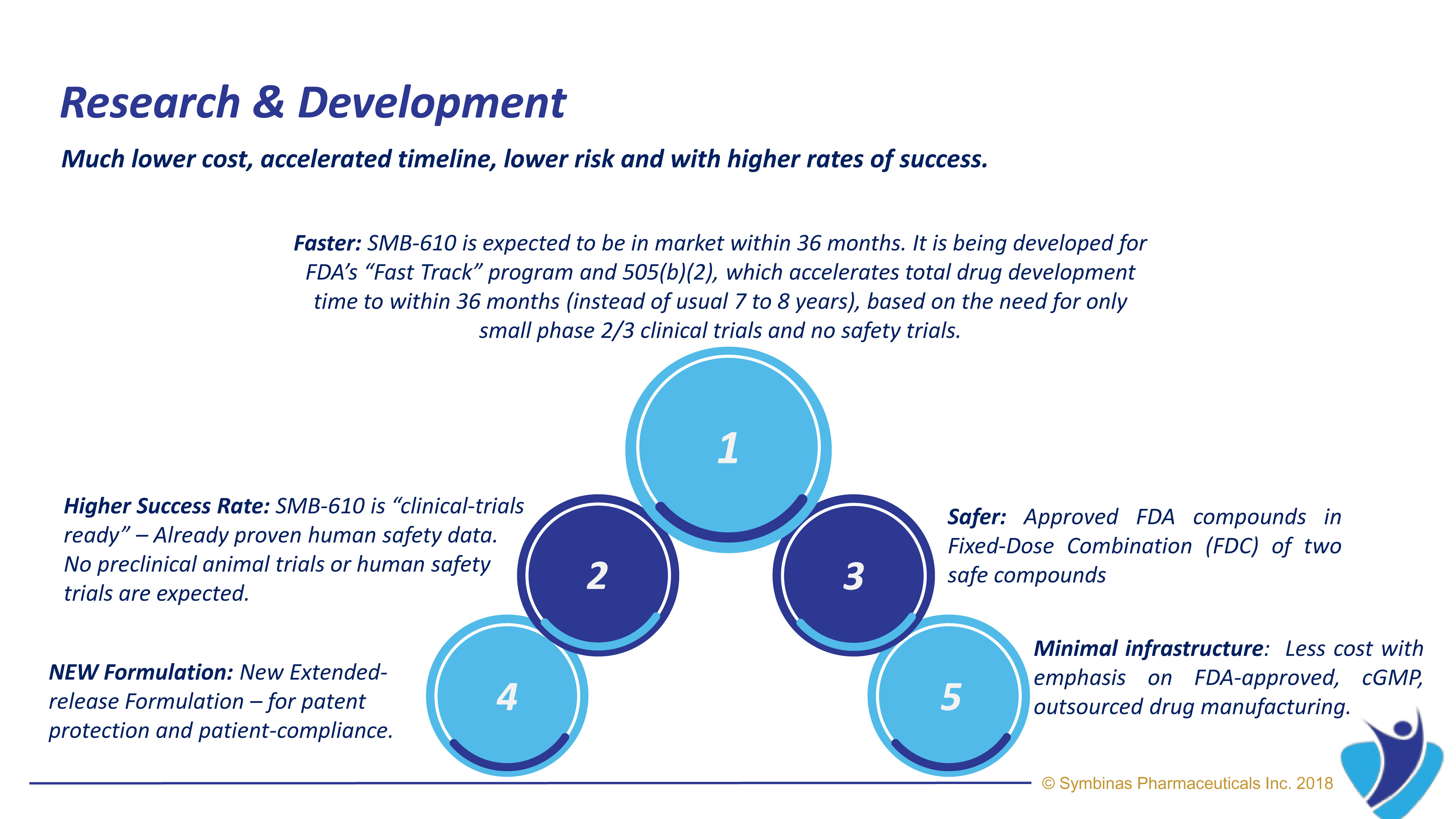
Symbinas’ strategy provides rapid and innovative therapies to deliver life-changing solutions in record time, at minimum cost, and with lower risk.
Symbinas’ LEAD product is SMB-603 to disrupt the global, $400 billion Concussion/TBI market. Using the FDA’s accelerated pathways, it has a target date within 24 months. It also has potential use in Alzheimer’s disease. Symbinas is also developing SMB-710 to treat chronic TBI. By impacting neuro-inflammation, SMB-710 has potential use in Alzheimer’s disease and Parkinson’s disease.
Our expansive pipeline includes drug candidates for the treatment of Concussion, Alzheimer’s Disease, Parkinson’s Disease, Depression and Schizophrenia.
Guided by our core purpose of positively impacting lives of patients and their families diagnosed with TBI, we incorporate unconventional approaches through strong partnerships with industry leaders, scientists, physicians and organizations engaged in helping patients with TBI. We work collaboratively across everything we do to achieve positive outcome in the lives of patients and their families.
OUR TEAM
Symbinas’s Team Includes Accomplished Product Development, Regulatory, Clinical And Scientific Visionaries In Pharmaceuticals, Stem Cells And Oligonucleotides With Proven Track Records Of Success.
Dr. Hardesh Garg
Founder, Chairman and CEO
Experienced purpose-driven physician and seasoned entrepreneur. Expert in Clinical Development, Regenerative Medicine and Stem Cells. A researcher, clinician and innovator in healthcare and life sciences. Well experienced in clinical trials, product development and regulatory affairs.

Dr. Jeffrey Cummings
Consultant Advisor, Clinical Programs
Professor of Medicine (Neurology) at Cleveland Clinic and Founding Director of Lou Ruvo Center for Brain Health in Las Vegas. Internationally renowned authority in clinical research and development in neurosciences.

Dr. Brent Masel
Consultant Advisor, Clinical Programs
Clinical Professor of Neurology, University of Texas Medical Branch in Galveston and National Medical Director at Brain Injury Association of America. Well renowned expert in concussion and TBI.

Tyler Currie
SVP, Strategic Communications
Diversified operations leadership and strategic partnerships in 30 countries, including previous executive roles in MedTech, professional sports and management consulting. Proven experience in athletic engagements with major leagues, international alliances and government relations.

Dr. Todd Schwedt
Consultant Advisor, Clinical Programs
Professor of Neurology at Mayo Clinic. He is Chair of Neurology Research at Mayo Clinic and board of trustees at International Headache Society. Well versed in concussion/TBI, clinical research and modeling brain imaging data.

Dr. Rodrigo Machado-Vieira
Consultant Advisor, Clinical Programs
Professor of Psychiatry at McGovern Medical School University of Texas Science Center in Houston. Proven expert in translational research, he was the Director of the Translational Research Clinic at the National Institute of Health (NIH).

Dr. Andrew Badley
Consultant Advisor, Clinical Programs
Professor of Infectious Diseases at world-renowned Mayo Clinic. Chair Mayo Clinic COVID Research task force and Chair of Molecular Medicine. Expert in infectious diseases and clinical Trials in COVID-19.

Dr. Barry Zingman
Consultant Advisor, Clinical Programs
Professor of Medicine at Albert Einstein College of Medicine, Clinical Director of Infectious Diseases at Montefiore Medical Center, Principal Investigator of NIH Adaptive COVID-19 Treatment Trial (ACT Trial), renowned authority in infectious diseases.

Dr. You-wen He
Scientific Advisor
Professor of Immunology at Duke University Medical Center. Expert in immunotherapy, vaccines, and molecular biology. Research areas include both innate and adaptive immunity against viral and bacterial infections as well as tumors.
THE TEAM: Collaborations
Legal Counsel:
- Thompson Hine (Corporate/Securities)
- Foley and Lardner (IP and FDA/Regulatory)
Finance & Accounting:
- Price Waterhouse Coopers LLP
Formulation Development, in-vivo and in-vitro tests:
- North Dakota State University, School of Pharmacy
Clinical Development and Trials: (Details Currently Under Negotiations)
- University of Texas at Houston, McGovern Medical School
- Cleveland Clinic Lou Ruvo Center for Brain Health at Las Vegas Nevada
- Temple University
- Princeton University
Business Partners, CDMO, CRO and Regulatory Affairs:
- FDA Filings – Established with a US based regulatory company
- Clinical Research Organization –a global CRO for Clinical Research
- Contract Drug Manufacturer – a global CDMO, specializing in pharma, generics and biotech